Introduction
Although expansion of chimeric receptor antibody (CAR)-T cells after infusion has previously been described, overt lymphocytosis with atypical morphology has not been widely reported in multiple myeloma (MM). Here we describe the kinetics of absolute lymphocyte count (ALC), its relationship with immune mediated toxicities, and clinical outcomes.
Methods
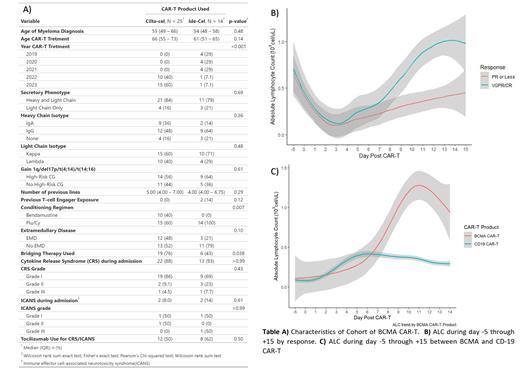
Thirty-nine patients (pts) receiving BCMA CAR-T (25 pts ciltacabtagene autoleucel (cilta-cel) and 14 pts idecabtagene vicleucel (ide-cel)) from two institutions were retrospectively reviewed. Data on baseline characteristics, presence of extramedullary disease (EMD), conditioning regimen, cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), severity of CRS/ICANS (according to American Society of Transplantation and Cellular Therapy (ASTCT) classification), ALC on day -5 through 14, time to best response (TBR), time to first response (TFR), depth of response, and outcomes were collected. Response was evaluated in pts with >3 months of follow up. Peripheral blood flow cytometry was performed in 3 cases at time of highest ALC. ALC data for 84 pts who received CD19 CAR-T (77 pts with axicabtagene ciloleucel and 7 pts with brexucabtagene autoleucel) for non-Hodgkin lymphoma (NHL) were included as a comparison. Maximum ALC (max-ALC) was defined as the highest ALC between day 0 and +15, baseline ALC was defined as the lowest ALC during day 0 through +3, and absolute ALC increase was defined as the difference of max-ALC and baseline ALC.
Results
Baseline characteristics of the cohort are described in Table A. Pts who received cilta-cel had earlier onset of CRS (median day +6 (IQR 2 - 8) vs +2 (IQR 1 - 2), p = 0.02), had higher max-ALC after CRS (median 1.7 cel/uL (IQR 0.8 - 5.3) vs 0.81 cel/uL (IQR 0.6 - 1.1), p = 0.02), and had a trend towards later max-ALC (median day +12 (IQR 11 - 13) vs +10 (IQR 9 - 13), p = 0.1). Baseline ALC did not differ by BCMA CAR-T product or number of previous lines of therapy. When compared to the NHL cohort, patients who received BCMA CAR-T had higher max-ALC (1.01 cel/uL (IQR 0.6 - 1.9) vs 0.4 cel/uL (IQR 0.2 - 0.7), p < 0.001) and this max-ALC occurred later (median day max-ALC +12 (IQR 10 - 13) vs day +10 (8 - 10), p < 0.01) Figure C.
Higher max-ALC and absolute ALC increase were associated with depth of response and occurrence of CRS/ICANS. Pts who had a deeper response (VGPR/CR) had a higher median max-ALC (1.2 cel/uL (IQR 0.77 - 1.81) vs 0.5 cel/uL (IQR 0.2 - 0.7) p < 0.01) and absolute ALC increase (1.2 cel/uL (IQR 0.7 - 1.8) vs 0.6 cel/uL (IQR 0.2 - 0.7) vs, p = 0.01) compared to those with PR or worse Figure B. Similarly, pts who did not progress on follow-up had higher median max-ALC (0.58 cel/uL (IQR 0.4 - 0.8) vs 1.3 cel/uL (IQR 0.9 - 1.9), p < 0.001) and absolute ALC increase (1.3 cel/uL (0.9 - 1.8) vs 0.6 cel/uL (0.5 - 0.8), p < 0.01) compared to progressors. Pts who experienced CRS had a higher absolute ALC increase i(1.05 cel/uL (0.7 - 2.7) vs 0.27 cel/uL (0.2 - 0.5), p = 0.02), and pts who had ICANS had higher max ALC (3.2 cel/uL (0.8 - 6.4) vs 1.01 cel/uL (0.6 - 1.8), p = 0.2). These results were consistent across BCMA CAR-T product, and there were no differences in baseline ALC across CAR-T product, response, disease progression, or number of previous lines. Furthermore, higher max-ALC was associated with higher odds of having VGPR/CR (OR 32.3 (2.23 - 240), p = 0.04) in univariable logistic regression. Flow cytometry data on 3 pts showed expansion of CD8+ T Cells during peak ALC with increase of CD8:CD4 ratio. Multicolor flow cytometry using an antibody BCMA at time of peak ALC showed expansion of CD4+ and CD8+ CAR-T, and further immunophenotypic characterization (differentiation, maturation, exhaustion) is currently being performed.
Conclusions
Post-CAR-T lymphocytosis was a common event after BCMA CAR-T therapy for MM, which presented a higher max-ALC, and at a later day when compared to CD19 CAR-T for NHL. Furthermore, ALC dynamics differed by BCMA CAR-T product, with cilta-cel exhibiting later CRS with higher max-ALC peaking at a later day when compared to ide-cel. Furthermore, higher max-ALC and absolute ALC increase were associated with deeper response (VGPR/CR) and sustainable response on follow up. Our findings suggest that peripheral lymphocytosis after CAR-T is disease specific (NHL vs MM) and post CAR-T lymphocytosis in peripheral blood is a potential biomarker for clinical outcomes and toxicity.
Disclosures
Monge:Janssen: Consultancy. Rosenbaum:Janssen Pharmaceuticals: Research Funding. Mapara:Incyte: Consultancy; Crispr/vertex: Consultancy; Bluebird bio: Consultancy. Lentzsch:Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; Caelum Biosciences: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: January 1, 2041; Celgene: Research Funding; Clinical Care Options: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Regeneron: Honoraria; Sanofi: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Reshef:TScan Therapeutics: Consultancy.